Mirrhia – Environmental Monitoring Solution
Jean Martin – CEO of Mirrhia
Mirrhia is specialized in the quality control of a drug. What does it mean? Well, it means that a patient in a hospital, a patient who swallows a drug, must be 100% certain that this drug has been manufactured under good conditions and that it is healthy.
To do this, there is a whole series of technologies that hospitals and the pharmaceutical industry must put in place. And we, at Mirrhia, are the ones who provide these technologies. This is done through our software, Mirrhia, which in fact industrializes this control and monitoring. It also involves equipment that must be installed. We have people in-house who install this equipment and calibrate it so that it works properly.
We have succeeded in transforming a company that did customized environmental monitoring projects into a company that offers an industrial solution for environmental monitoring on a global scale. It is a complex journey because you must move from know-how to an IT system. This requires the evolution of our people. It alqo requires an evolution of the entire company structure.
This decision was made by investing in the product. It has involved the creation of a distribution network and a partnership, for example with the bioMérieux group in Lyon, which is an essential partner for us.
The future is to continue this approach and to become one of the top three companies producing environmental monitoring software worldwide. And as this sector moves continuously, innovation is the key to success. To position ourselves in this top 3 and to stay there, we will continue to invest heavily in R&D.
Nicolas Gilson – Sales and Marketing Director
First and foremost, Mirrhia meets a regulatory obligation, an imposition that is found in the European directives known as GMP, good manufacturing practices. And we will find authorities such as the AFMPS (aka the federal agency for medicines and health products) which will ensure that we meet these requirements.
Today, we have, especially in the pharmaceutical sector, a lot of processes that are still very manual, very laborious, which in fact still require a lot of interaction. For example, we transcribe things onto paper, which takes time and generates errors. So, we’ll talk about false positives, i.e., thinking that a room is contaminated when it isn’t, which can have major impacts. Or we’ll talk about false negatives, we’ll think that the room is not contaminated when in fact it is and obviously, this is more serious. Potentially, your medicine will be impacted while you think it is safe.
These processes, which are now extremely manual, will in the near future be completely digitized from A to Z, with support for the operator. He will have a tablet on which he can view his process. And then he will also be able to scan his consumables, scan the equipment he is going to work with. He will be able to authenticate himself on the system, which means that there will be a complete traceability. And the result of this traceability is, above all, that from this digitalization, we will have almost real-time information on the contamination of the room.
To talk about the near future for Mirrhia… We already have international coverage that we would like to extend as much as possible. We are present in the United States, we are present in Singapore, in Europe, of course etc. We would like to continue to expand our geographical presence. There is also a sectoral evolution. We are very present in the pharmaceutical world and in hospitals. Tomorrow, we would like to grow in industries such as aerospace, food processing and cosmetics.
And then, if we look a little further, we are going to move towards Big Data, Artificial Intelligence, the capacity to anticipate as much as possible the behavior of a room to be able to take preventive actions rather than reactive actions based on artificial intelligence.
More information on Mirrhia
- Send us your message, question or comment. Our team will get back to you shortly.
A video of Jean & Nicolas
Want to see our CEO & Managing Director in action?

Advanced Automation in Cleanrooms
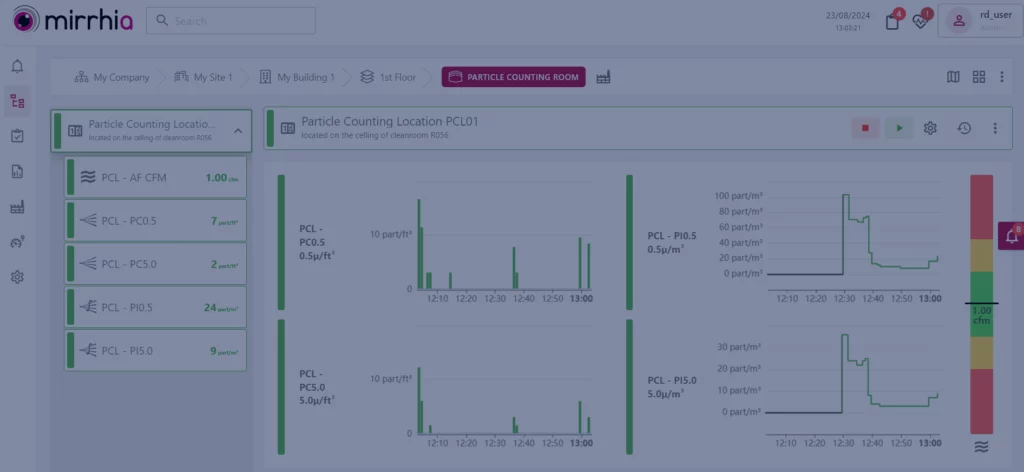
Mirrhia 2.5 is there!

Omega, Mirrhia’s Italian partner, in the spotlight in Genova Impresa

Arrival of Etienne Van den Bogaert as Managing Director
Mirrhia 2.4 is coming

Data Integrity in The Pharmaceutical Industry

Laborama 2024

Laboratory Temperature & Humidity Monitoring
