Data Integrity in The Pharmaceutical Industry
Understanding what data integrity is in the pharmaceutical world is not that simple, but it makes perfect sense when we know that in the end, it is our health to all that is at stake!
I will therefore try to give you the key elements to understand what data integrity is and what it is for.
In a context where regulation and the need to meet strict standards are omnipresent, it is essential to ensure the integrity of the data collected. This data plays a vital role in process validation, product traceability and compliance with regulations such as Annex 1, CFR 21 Part 11 and the ALCOA concept.
1. Data integrity – What is it?
Data integrity in pharmaceuticals refers to the quality and reliability of data used in all stages of drug development, production, quality control and distribution.
2. Data integrity – Regulations
2.1. Annex 1: Requirements for sterile manufacturing environments in the pharmaceutical industry.
Annex 1 forms a central pillar of pharmaceutical regulations as it sets out specific requirements for sterile manufacturing environments within the pharmaceutical industry. These requirements encompass various aspects such as:
- Design and monitoring of installations
- Cleaning and disinfection procedures
- As well as process validation.
The main objective of Annex 1 is to minimize the risks of microbiological contamination, thereby ensuring that your production of sterile medicinal products will be of high quality and meet the highest safety standards.
To comply with Annex 1, your pharmaceutical company must implement strict environmental monitoring measures, including particulate and air quality monitoring, microbiological monitoring and process validation.
These measures aim to ensure that your production environment is sterile and safe, which is essential for the manufacture of injectable, ophthalmic medicines and other products sensitive to contamination.
2.2. FDA CFR 21 Part 11 guidance on the use of electronic signatures and management of electronic records.
CFR 21 Part 11, issued by the United States Food and Drug Administration (FDA), is a set of guidelines for the use of electronic signatures and management of electronic records in the pharmaceutical industry. This regulation aims to guarantee the integrity, authenticity and reliability of your electronic data associated with the manufacturing, control and distribution of your pharmaceutical products. Under 21 CFR Part 11, your pharmaceutical company is required to implement compliant electronic systems capable of ensuring full traceability of your processes and critical decisions throughout the lifecycle of your product.
Compliance with 21 CFR Part 11 therefore requires that you adopt strict electronic data management practices, particularly with regard to the identification, authentication and validation of your users, as well as the securing and retention of electronic records. This regulation clearly has an impact on reducing the risks of falsification or manipulation of data, while promoting the transition to more efficient and secure electronic documentation systems.
And that’s beautiful but above all it’s essential!
2.3. ALCOA: Fundamental principles for ensuring data integrity and reliability.
The ALCOA principles provide an essential framework for ensuring data integrity and reliability in the pharmaceutical industry.
ALCOA, which stands for Assignable, Legible, Compliant, Original and Accurate, sets clear standards for the documentation and retention of your data throughout the pharmaceutical process:
- Your data must be attributable, meaning it must be associated with a specific user or process, to ensure traceability and accountability.
- They must be readable, that is to say clear and understandable to avoid any ambiguous interpretation.
- Additionally, your data must comply with current standards and regulations, ensuring regulatory compliance.
- They must also be original, that is, they must be recorded at the time the event or observation took place, without subsequent alteration or manipulation.
- Finally, your data must be accurate, accurately reflecting the information collected or generated.
By following these ALCOA principles, your pharmaceutical company can maintain data integrity throughout the lifecycle of your products, building confidence in the quality and safety of pharmaceutical products.
3. Mirrhia and data integrity
3.1. Mirrhia and compliance with standards
Mirrhia offers you an innovative platform that meets the specific needs of the pharmaceutical industry:
- Firstly, Mirrhia offers a complete data management solution, enabling the collection, storage and easy access to all environmental data in an efficient and secure manner.
- Our platform offers a user-friendly interface, making it easy to enter and view data, as well as generate personalized reports that can respond to any audit.
- Mirrhia also stands out for its rigorous compliance with current standards and regulations, including Annex 1, CFR 21 Part 11, and the ALCOA principles, all cited above. Our software is designed to ensure data traceability and integrity, ensuring compliance with regulatory requirements.
- Mirrhia integrates advanced security mechanisms, such as user access management and modification traceability, to prevent any unauthorized alteration or manipulation of data.
3.1.1. Focus on Annex 1
As an advanced environmental monitoring system, Mirrhia offers specific features that enable your pharmaceutical company to comply with the strict Annex 1 requirements.
Mirrhia facilitates the real-time collection of critical environmental data such as air quality, suspended particulate matter and microbial contamination, providing a detailed view of the status of the production environment.
Mirrhia also offers powerful analysis and reporting tools that allow you to quickly and accurately assess the compliance of your production environments with Annex 1 standards.
The platform also allows for automated reporting, simplifying your process of documenting and tracking corrective actions needed to maintain regulatory compliance.
3.1.2. Focus on 21 CFR Part 11
Mirrhia demonstrates its commitment to meeting the highest standards set by the United States Food and Drug Administration (FDA) for the management of electronic data and electronic signatures by complying with 21 CFR Part 11. This compliance ensures that Mirrhia is equipped with the necessary features to guarantee the integrity, authenticity and traceability of your environmental data.
Mirrhia offers advanced security mechanisms to control access to data, guarantee its confidentiality and prevent unauthorized alteration. The software makes it easy to generate reports and document processes, ensuring transparency and complete traceability of your operations.
Thanks to this compliance with 21 CFR Part 11, you can rest easy about the reliability and integrity of your environmental data and also the preservation of your company’s reputation.
3.1.3. Focus on Gamp 5 Category 4
The GAMP 5 Category 4 classification, established by the International Society for Pharmaceutical Engineering (ISPE), recognizes Mirrhia’s compliance with the most recent Good Manufacturing Practice (GMP) guidelines for computerized systems. This classification guarantees that Mirrhia meets the highest standards in the design, development, implementation and maintenance of computerized systems used in the pharmaceutical industry.
3.1.4. Focus on ALCOA
Mirrhia is resolutely committed to respecting the ALCOA principles (Assignable, Legible, Compliant, Original and Accurate), fundamental to guaranteeing the integrity of data in environmental monitoring.
By integrating ALCOA requirements into its design and operation, Mirrhia ensures complete data traceability, allowing each data point to be traced back to its original source. The data collected and managed by Mirrhia are therefore attributable to their origin, readable, recorded at the time of their creation, original and precise, thus meeting the strictest standards in terms of data integrity.
4. Mirrhia features that meet the data integrity guarantee
Our Mirrhia EMS is a feature-packed, essential solution that helps ensure environmental data integrity across various industry sectors, including pharmaceuticals. Here are some key features of an EMS that help ensure this data integrity:
4.1. Automated data collection
Mirrhia is capable of automatically collecting your environmental data from sensors and monitoring instruments distributed throughout facilities, in real time. This ensures the accuracy and consistency of the data collected.
4.2. Secure data storage
Mirrhia ensures secure storage of your data, using robust security protocols to protect sensitive information from alteration or unauthorized access.
4.3. Data traceability
Mirrhia provides complete traceability of your data, recording every step of the collection, storage and analysis process. This allows you to trace the origin of the data and guarantee its integrity.
4.4. Managing access and authorizations
Mirrhia allows you to manage your users’ access and authorizations in a granular manner, limiting access to data only to authorized people. This helps prevent unauthorized manipulation of data.
4.5. The audit trail
Mirrhia generates a detailed audit trail of all data-related activities, including changes made, accesses made, and actions performed by your users. This allows all interactions with the data to be tracked and analyzed.
4.6. Custom reports
Mirrhia allows you to generate personalized reports on your environmental data, adapted to the specific needs of users and regulators. These reports are clear, complete and comply with regulatory requirements. You can generate them manually or automatically with periodic sending. It’s magical 😊
5. Mirrhia’s technological innovations to strengthen data integrity
5.1. Electronic signature
Electronic signature plays a crucial role in data authentication and traceability, providing a secure and reliable way to attest to the origin and integrity of electronic information.
First of all, it allows you to uniquely identify the author of a document or an action, thus guaranteeing the authenticity of the data and the responsibility of the user. This is essential in regulated industries such as pharmaceuticals, where you need to be able to guarantee the traceability of actions and decisions that have been taken.
Additionally, the electronic signature ensures non-repudiation, meaning that once it is affixed, the author cannot deny having performed the signature or the associated action. This builds confidence in the integrity of the data and prevents further tampering or alteration.
Traceability is also improved thanks to the electronic signature, because it makes it possible to precisely follow and identify the different stages of the process, from creation to validation of data.
And of course, electronic signature is a feature within Mirrhia.
5.2. The Four Eyes Principle – Strengthening the validation of critical data
The “4 eyes” control is a recommended or even mandatory practice in many fields, including the pharmaceutical industry, where the validation of critical data is crucial to guarantee the quality and safety of your products. This feature implies that an action or decision can only be validated after it has been reviewed and approved by at least two independent people.
In the context of validating critical data, the “4 eyes” control offers you an additional level of security and quality assurance. By requiring double-checking of all critical data before validation, this practice significantly reduces the risk of unintentional errors or tampering. Additionally, it promotes collaboration and communication within the team, encouraging the sharing of knowledge and perspectives among members.
A question about data integrity in the pharmaceutical industry?
Do not hesitate to contact us now.
Mirrhia 2.5 is there!

Omega, Mirrhia’s Italian partner, in the spotlight in Genova Impresa

Arrival of Etienne Van den Bogaert as Managing Director
Mirrhia 2.4 is coming

Laborama 2024

Laboratory Temperature & Humidity Monitoring

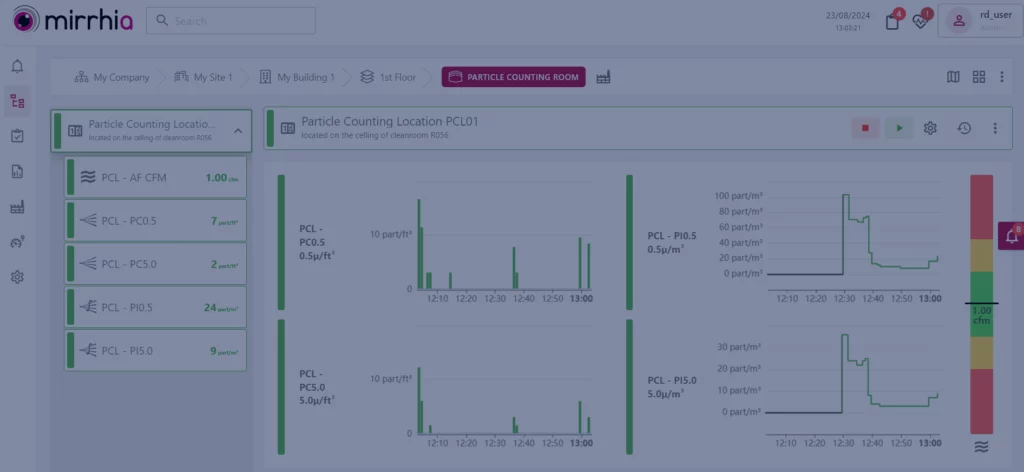
Particle Counters | Case Studies & EMS in Pharma

Particle Counting for Pharma
