Particle Counters | Case Studies & EMS in Pharma
And so this ends this wonderful journey dedicated to the world of particle counters. I don’t know about you, but I feel a sense of excitement.
I hope it is now as clear to you as it is to me that these small but powerful devices are revolutionizing the pharmaceutical industry. And then we, at Mirrhia, are obviously proud to be able to integrate almost all brands of particle counters into our Environmental Monitoring System and to help our clients in their Contamination Control Strategy.
To conclude, I have prepared some fictitious case studies for you as well as an overview of the trends in particle counters. Finally, I will present to you what is expected tomorrow in terms of innovation in the magical world of particle counters.
1. Case Studies
Case Study 1: Optimization of Aseptic Filling Line
Challenge
A pharmaceutical company struggled to maintain required cleanliness levels in its aseptic filling line, a crucial area for product quality and patient safety.
Solution
The company integrated particle counters into its aseptic filling line to monitor airborne particle contamination in real-time. They established baseline particle counts and implemented continuous monitoring protocols.
Impact
- Early detection of contamination events allowing for immediate corrective actions.
- Reduced risk of product rejection due to particle contamination.
- Demonstrated compliance with regulatory standards, enhancing product quality and patient safety.
Case Study 2: Sterile Preparation Facility Upgrade
Challenge
The facility implemented a comprehensive environmental monitoring system, including airborne particle counters. Real-time data was integrated into the process control framework.
Solution
The company integrated particle counters into its aseptic filling line to monitor airborne particle contamination in real-time. They established baseline particle counts and implemented continuous monitoring protocols.
Impact
- Improved real-time monitoring and control of environmental conditions.
- Enhanced regulatory compliance and reporting capabilities.
- Streamlined validation processes and reduced downtime for maintenance.
Case Study 3: Cleanroom Expansion Project
Challenge
A pharmaceutical manufacturer embarked on a cleanroom expansion project to meet increased production demand, requiring strict adherence to cleanliness standards.
Solution
Particle counters were strategically placed in the expanded cleanroom, and data was integrated into the existing process control system. Calibration and validation procedures were established.
Impact
- Ensured the expanded cleanroom maintained required cleanliness levels.
- Facilitated seamless integration with existing process control systems.
- Provided critical data for regulatory compliance and validation of the expansion project.
Case Study 4: High-Potency Drug Manufacturing
Challenge
A facility producing high-potency drugs faced challenges in controlling airborne particle contamination due to the increased sensitivity of the products.
Solution
The facility implemented particle counters designed for ultrafine particle detection. Sampling points were strategically located near critical process areas.
Impact
- Achieved stringent cleanliness standards for high-potency drug manufacturing.
- Mitigated the risk of contamination events, preserving product quality.
- Demonstrated a high level of environmental control to regulatory authorities.
2. Particle Counters and EMS in pharma
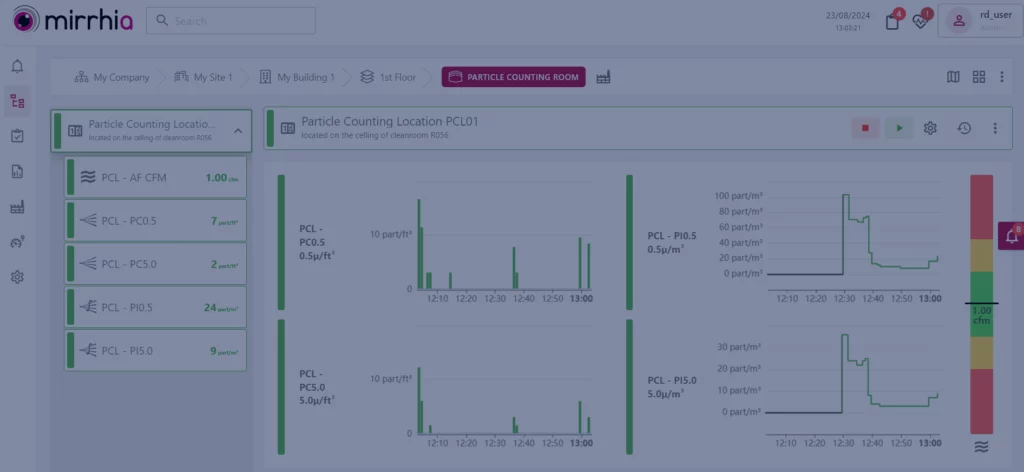
Environmental Monitoring Systems (EMS) ensure the cleanliness and safety of controlled environments, especially in industries like pharmaceuticals, healthcare, and manufacturing. Particle counter stands out as an important component for achieving the highest standards of contamination control.
As mentioned earlier, particle counters are sophisticated instruments meticulously designed to measure the concentration of particles in the air. These particles, often imperceptible to the naked eye, can be critical contaminants in highly sensitive environments. Hence, the imperative for having particle counters operational in your critical zones is undeniable.
At Mirrhia, we take pride not just in providing technological advancement but in offering a singular, comprehensive solution. Together with other critical parameters (such as temperature, humidity, pressure differentials, etc., the integration of particle counters into our EMS goes beyond being a mere advancement—it’s a necessity for maintaining exemplary standards of environmental cleanliness and compliance.
This integration ensures real-time data collection, allowing for an immediate response to contamination events. The continuous data collection facilitates trend analysis, enabling the proactive identification of potential issues before they escalate. This aspect becomes particularly crucial when producing sterile medication, where maintaining stringent cleanliness standards is paramount.
What sets our approach apart is the commitment to a standardized framework. The integration of various brands seamlessly into the Mirrhia EMS enhances the system’s adaptability, making it a versatile solution catering to the diverse needs of different industries. This standardized approach not only streamlines operations but also provides a unified and consistent reporting structure—a critical element in ensuring clarity and efficiency in contamination control strategies.
The integration of particle counters into Mirrhia’s EMS is not just a technological feat; it’s a strategic move towards offering a single, standardized solution. This approach not only meets the highest industry standards but also ensures adaptability and clarity in the pursuit of environmental cleanliness and compliance.
A question on Particle counter?
Don’t hesitate to contact us now. Send us a message and we’ll get back to you shortly.
Mirrhia 2.5 is there!

Omega, Mirrhia’s Italian partner, in the spotlight in Genova Impresa

Arrival of Etienne Van den Bogaert as Managing Director
Mirrhia 2.4 is coming

Data Integrity in The Pharmaceutical Industry

Laborama 2024

Laboratory Temperature & Humidity Monitoring

Particle Counting for Pharma
