EMS system in pharma
The Crucial Role of an Environmental Monitoring System (EMS) in the pharmaceutical Industry
In the captivating world of the pharmaceutical industry, precision and reliability are non-negotiable. As you can imagine, the production of sterile medications demands exceptionally rigorous control at various levels. The level we’re concerned with is, of course, environmental conditions. Environmental monitoring plays a pivotal role in upholding the strictest standards of quality and safety. This is serious business, and rightfully so.
In this article, I’ll shine a spotlight on the EMS, specifically in the pharmaceutical sector. I’ll outline the primary reasons for its existence, highlight key parameters requiring specific monitoring, illustrate potential consequences in the absence of this surveillance system, and finally, walk through the crucial steps in the production of sterile medications, along with a concrete example of parameters to monitor at each stage.
Are you ready?
The EMS – Cornerstone of Compliance
The Imperative of Sterility
First things first, what is sterile medication? It’s a medication prepared and packaged to be free from any form of microorganisms such as bacteria, fungi, viruses, and spores. This means it contains no microbial contaminants that could potentially be harmful to the patient (that’s all of us).
And what about Sterility?
Sterility is particularly crucial for medications administered through direct injection into the body (known as injectables), as any presence of microorganisms could lead to severe infections in the patient. Hence, sterile medications are manufactured in highly controlled conditions, usually in specialized pharmaceutical facilities equipped with cleanrooms. You can bet these rooms are beyond spotless.
Achieving and maintaining sterility is a paramount requirement to ensure the safety and efficacy of these pharmaceutical products. The EMS serves as the linchpin in adhering to these strict yet necessary standards in the production of sterile medications.
Assuring Quality and Product Safety
As I’ve emphasized before, an EMS (Environmental Monitoring System) plays a pivotal role in guaranteeing the quality and safety of sterile medications by monitoring the environment in which they are manufactured.
Here are some ways an EMS contributes to this:
Detecting Potential Contaminations: An EMS continuously monitors parameters like airborne particle concentration, temperature levels, humidity, differential pressure, etc. This allows for swift detection of any deviations from established standards, which could indicate a potential contamination of the production environment. And of course, that’s precisely what we aim to prevent…contamination.
Preventing Microbial Contaminations: By monitoring the presence of microorganisms in the air and on surfaces, an EMS ensures that the production environment remains sterile. If microbial contaminants are detected, corrective measures can be taken immediately.
Regulatory Compliance and Data Integrity
By now, you’re probably familiar (or perhaps you’re a seasoned pro) with the fact that in the pharmaceutical industry, adhering to strict regulatory standards isn’t a choice—it’s a legal obligation. Regulatory bodies like the FDA (Food and Drug Administration) and the EMA (European Medicines Agency) have stringent guidelines regarding data integrity and product quality. An EMS is an indispensable tool in demonstrating compliance with these standards.
Audit Trail:
- The audit trail securely and chronologically records all activities and modifications made in the system.
- It allows tracking of who performed a specific action, when it was done, and which data was modified.
This ensures transparency and data integrity, both of which are essential for regulatory compliance.
So, basically, you can’t do what you want when you want! That’s normal!
Data History:
- Data history refers to the system’s ability to store and provide access to a comprehensive history of collected data over a specified period.
- This allows tracking the evolution of environmental parameters over time, which is crucial for trend analysis, anomaly detection, and informed decision-making.
- It can also be used to fulfill requests for historical reports from regulatory bodies.
Data Security:
- EMS data must be securely stored to prevent unauthorized access or alteration of information.
- Security measures such as authentication, encryption, and access control are essential.
Report Generation:
- Having access to data is good. Being able to generate customized reports from collected data is even better.
- These reports can be used to document regulatory compliance, for internal and external communication, as well as for audits and inspections.
- They should be customizable based on the specific needs of the company and regulatory requirements.
EMS or Critical Parameter Monitoring
Temperature and Humidity
Precise control of temperature and humidity levels is crucial in the production of sterile medications. Deviations in these parameters can compromise the stability and effectiveness of your product. An Environmental Monitoring System (EMS) continuously monitors and alerts you to any deviations, allowing for immediate corrective action.
Imagine a scenario where a pharmaceutical manufacturer lacks an environmental monitoring system. In this case, temperature fluctuations could occur without detection. This could lead to the degradation of sensitive compounds, rendering the product ineffective or even harmful to patients.
Air Quality and Particles
Maintaining an environment free from contaminants is of utmost importance. It’s a bold statement, but it’s true. And the EMS is there to assist you by overseeing air quality and particle levels, ensuring that your production area complies with specified cleanliness standards. This is particularly crucial in clean rooms and controlled environments.
In the absence of an EMS, contaminants could infiltrate the production environment. This scenario poses a significant risk to the sterility of pharmaceutical products, potentially leading to large-scale product recalls and damage to the company’s reputation. If it’s your company, you’d be in quite a pickle.
But hey, Mirrhia is here.
Pressure Differentials
In sterile environments, maintaining appropriate differential pressures is central to preventing the penetration of contaminants. The EMS notifies you of any deviation that could compromise the integrity of your sterile zone. It’s fabulous, isn’t it?
There are, of course, other parameters you can monitor! But hey, we won’t list them all.
EMS in Sterile Medication Manufacturing
The production of sterile medications is a complex and highly regulated process, essential to ensuring the effectiveness and safety of pharmaceutical products. I know I’m repeating myself, but it’s just that important.
The role of environmental monitoring is fundamental in this process, ensuring that manufacturing conditions remain optimal throughout production.
Let’s explore the key stages of sterile medication manufacturing, with a particular focus on the environmental monitoring system and its role.
Preparing Raw Materials
Before delving into the actual manufacturing process, it’s crucial to ensure the quality of raw materials. These must be carefully selected and tested to ensure compliance with pharmaceutical standards. Environmental monitoring already plays a role at this stage by monitoring the storage and handling conditions of raw materials, controlling temperature, humidity, and other environmental parameters.
Concrete Example: If raw materials are exposed to inadequate temperature and humidity conditions, it could compromise their integrity and alter the quality of your final product. Insulin is a hormone naturally produced by the pancreas that regulates blood sugar levels. Insulin is a complex and delicate protein that can be denatured (meaning it loses its three-dimensional structure) or degraded by variations in temperature, humidity, or exposure to light. Therefore, its storage and transport must be carefully controlled to maintain its effectiveness.
Formulation and Mixing
Once the raw materials are approved and have arrived at their destination, they are formulated and mixed according to specific protocols. This step requires particular attention to avoid any contamination. Environmental monitoring steps in by overseeing air cleanliness, controlling levels of suspended particles, and verifying the absence of potentially harmful microorganisms.
Concrete Example: High-efficiency air filters are used to maintain a clean environment, and particle counters are placed to monitor particle levels in formulation rooms.
Aseptic Packaging
Once the medication is sterilized, it must be aseptically packaged to prevent any subsequent contamination. Environmental monitoring plays a crucial role here by ensuring that packaging rooms remain sterile, controlling particle levels, and monitoring differential pressure to prevent the infiltration of unfiltered air.
Concrete Example: Packaging rooms are equipped with laminar flow ventilation systems that create a unidirectional environment, preventing contamination.
Complete Traceability
We’re back to the Audit trail or audit journal. Throughout the manufacturing process, it’s imperative to trace each step and each batch of product. This means meticulously recording and documenting production details, test results, and environmental conditions. This allows for complete traceability, which is essential to meeting quality and safety standards.
Concrete Example: Packaging rooms are equipped with laminar flow ventilation systems that create a unidirectional environment, preventing contamination.
What happens if you cannot prove that your sterile medication was not manufactured under proper conditions?
- If you cannot provide evidence through a report that you adhered to the environmental conditions for your sterile medication, it can have serious consequences such as:
- The suspension or revocation of drug approval: The market authorization for the sterile medication could be suspended or revoked, meaning it would no longer be legally available for sale or distribution.
- Fines and penalties: You may be subject to fines and other financial penalties due to non-compliance with regulations.
- Legal liability: If patients suffer harm due to non-compliance with environmental standards, you could be held legally responsible and face lawsuits.
- Consequence for your company reputation: Your company’s reputation could be severely impacted, potentially leading to long-term consequences for consumer and healthcare professional trust.
It is therefore crucial to strictly adhere to environmental regulations and meticulously document procedures to ensure compliance and safety of sterile medications. If you have specific doubts or concerns, it is recommended to consult a pharmaceutical regulatory expert or contact the relevant regulatory agency for advice tailored to your situation. It’s a safer choice!
EMS for the Pharma Industry – It’s MIRRHIA FACILITY®
And at Mirrhia, we’ve developed a solution to monitor the specific environmental conditions for the production of sterile medications. This solution is MIRRHIA FACILITY®.
If you want to learn more or discuss your project in your pharmaceutical company with us, contact us now!
A Minute With Marie
Want to see the series “A Minute with Marie” on EMS in Pharma?

Advanced Automation in Cleanrooms
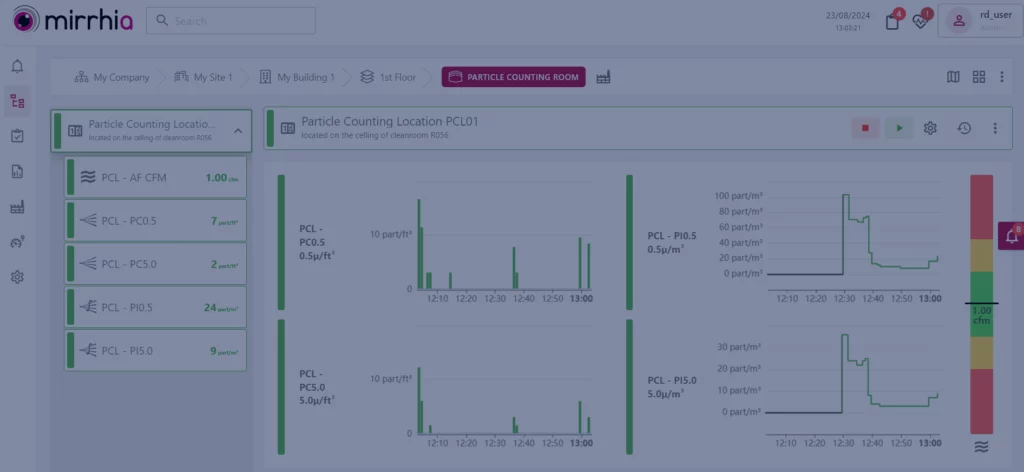
Mirrhia 2.5 is there!

Omega, Mirrhia’s Italian partner, in the spotlight in Genova Impresa

Arrival of Etienne Van den Bogaert as Managing Director
Mirrhia 2.4 is coming

Data Integrity in The Pharmaceutical Industry

Laborama 2024

Laboratory Temperature & Humidity Monitoring
