Keep your LABGUARD® equipment (L2 & 3D)
You may have already learned that bioMérieux announced the end of sales of LABGUARD® 2 solution at the end of 2020 and LABGUARD3D at the end of 2022 (end of customer service at the end of 2023).
This news positions Mirrhia as the provider of choice to ensure not only the sustainability of LABGUARD® 3D equipment thanks to the flexibility of its software, but also shows bioMérieux’s trust in Mirrhia.
But Mirrhia didn’t stop there. Thanks to the diligent work of its R&D team and the integration of additional developments, Mirrhia also offers its customers the possibility of keeping their LABGUARD® 2 equipment.
With Mirrhia, get the most of your EMS
Mirrhia is an independent environmental monitoring solution. Our system is compatible with most brands of probes and measurement equipment including LABGUARD® equipment.
Mirrhia is a validated, configurable, scalable and open monitoring system that controls all your critical parameters.
Ensure your business continuity with Mirrhia
Since the announcement by bioMérieux, Mirrhia has deployed every effort to make the transition as smooth as possible for users. We are proud today to announce that we are ready to support you in this migration.
Mirrhia brings together a unique set of features on one platform:
- Real-time monitoring
- Alarm management & notification
- Qualified report and audit trail
- Tree structure and 2D visualization
- User management (LDAP)
- Calibration management
- Electronic Batch Record
- Predictive maintenance
- Task management, etc.
Compatibility of Mirrhia with your equipment
Mirrhia’s open architecture allows data acquisition from any type of equipment, sensors or probes.
Mirrhia has been designed to monitor all your installations:
- Clean rooms & warehouses
- Laboratories / QC
- Preparation laboratories
- Filling lines
- Blood / tissue banks
- Clinical pharmacies
- Isolation & surgery rooms
- Fertility Center …
Make your LABGUARD® equipment profitable
With Mirrhia, make the most of your EMS and capitalize on your investment by keeping all your equipment.
Commissioning of our Mirrhia solution
As our approach is based on a risk analysis, Mirrhia provides you with pre-qualified software to which we associate IQ/OQ protocols adapted to your installations.
Conformité de notre logiciel aux réglementations
Keep complete control of your environment while reponsding to your local regulatory requirements.
Mirrhia, a global environmental monitoring solution
Software
A simple and risk-free migration in 3 steps:
- Installation & configuration of Mirrhia
- Switch from LABGUARD® equipment to Mirrhia
- Migrating your historical data
Equipment
- Keep your LABGUARD® equipment
- The extension of your park and the integration of your new equipment
Support et service
- The support is available in 3 formulas including direct access to Mirrhia’s hotline.
- Calibration: Mirrhia provides calibration for all types of probes.
- Cartography according to the standards in force.
Plan your transition with Mirrhia now!
Discover all the benefits of migrating to our Mirrhia software with our LABGUARD® experts.
Mirrhia meets all of your needs now and in the future for 25 years.
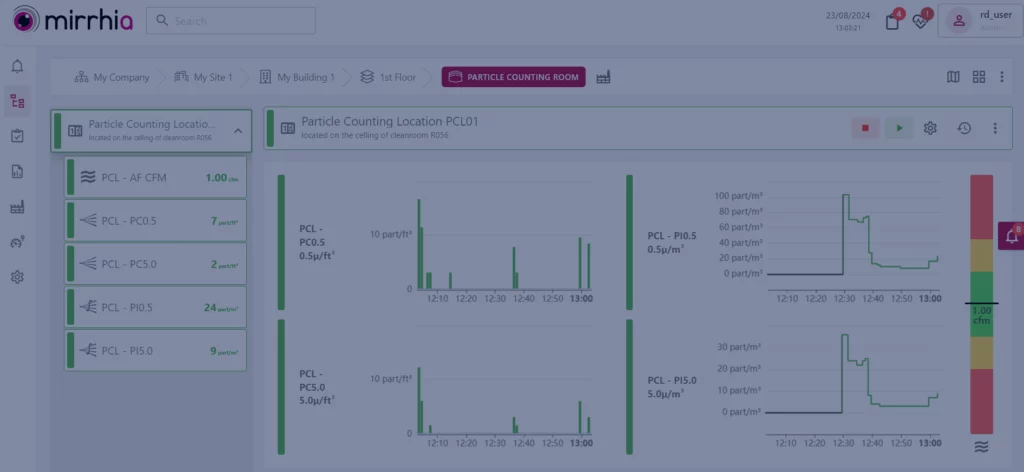
Mirrhia 2.5 is there!

Omega, Mirrhia’s Italian partner, in the spotlight in Genova Impresa

Arrival of Etienne Van den Bogaert as Managing Director
Mirrhia 2.4 is coming

Data Integrity in The Pharmaceutical Industry

Laborama 2024

Laboratory Temperature & Humidity Monitoring

Particle Counters | Case Studies & EMS in Pharma
