The Annex1 for Dummies
When you are new to the environmental monitoring system business, you quickly hear people around you talking about Annex 1… and that is normal. The EU (EudraLex which is a collection of rules applied to medicinal production in the EU) GMP (Good Manufacturing Practice) Annex 1 contains guidelines for the manufacturing of sterile products.
The first issue of Annex 1 was released in 1989. There has been lots of partial improvements afterwards but not a full revision of the document. The last improvement was made in 2009. After that, proposals for revision were made and different drafts were released for comments.
The last draft (draft 12) has been released in February 2020. The good news is we might see a final version of the document end of 2021 with an implementation in 2022.
What does the Annex1 contain exactly?
There are 11 chapters in the Annex 1. It starts with its scope and principles followed by a chapter on Pharmaceutical Quality Management (PQS). Then it lists the premises it applies to, the equipment and utilities. Further comes a chapter on personnel and a next one that covers production and specific technologies. Chapter 9 talks about viable and non-viable environmental and process monitoring followed by Quality Control. And finally, you will find the glossary.
The 2 main topics in the Annex 1 are Quality Risk Management (QRM) and Contamination Control Strategy (CCS). Those topics are closely linked to facilities’ environmental monitoring.
What is Quality Risk Management (QRS)?
Actually, the whole annex 1 is about Quality Risk Management. Risk Management is a systematic process for the assessment, control, communication, and review of risks. It ensures the highest quality level of a product to the patient. On top of it, Quality Risk Management improves decision making if a problem occurs in terms of quality.
There are a few Risk management tools recognized in the pharmaceutical industry and by regulators (as described in ICH Q9 guidelines):
- Failure Mode Effects Analysis (FMEA)
- Failure Mode, Effects and Criticality Analysis (FMECA)
- Fault Tree Analysis (FTA)
- Hazard Analysis and Critical Control Points (HACCP)
- Hazard Operability Analysis (HAZOP)
- Preliminary Hazard Analysis (PHA)
What it a Contamination Control Strategy (CCS)?
A contamination control strategy should address the different elements of the process design that will protect your product from any contamination, such as raw material control, process risk assessment, preventive maintenance, cleaning disinfection or monitoring systems to name a few.
A contamination control strategy should be designed to help manufacturers identifying and resolve risk. It requires a solid understanding and knowledge of the product and design process to be able to assess any risk or hazards that may occur and intervene.
It’s an ongoing process that need to be maintain and reviewed regularly once it is implemented to make sure products are always manufactured in accordance with the CCS and GMP.
What about Monitoring in CCS?
Environmental Monitoring is crucial in the contamination control strategy and is used to monitor and determine the level of contamination in a cleanroom environment or manufacturing area.
Environmental monitoring provides data on contamination control and helps predicting any problems or taking proactive decision to avoid contamination.
Quality Risk Management helps to determine when, where and on what monitoring should be done! Room qualification grades gives you an insight on the level of monitoring it must have. The surfaces and the location in the facility, air environment, materials, and personnel (ex: sample on hand gloves and gowns) must also be considered.
Full Knowledge of the critical points of the facilities is required.
What can we expect of the revision of Annex 1?
The new revision of Annex 1 is going to request manufacturers to re-think their cleanrooms layout, with new approaches to the design of environmental monitoring plans of the critical areas, changing rooms, HVAC system and cleanroom qualification paying strong attention to Pharmaceutical Quality System (PQS) and Quality Risk Management as described in the guidelines ICH Q9 and ICH 10 guidelines respectively.
It is time for Annex1 to get a review. It needs to align with the industry expectations and technologies. Some key requirements need to be clarified. It also needs to be aligned with ICH Q9 and Q10.
The new version of Annex1
Yeap, it has been released so here it is, just for you.
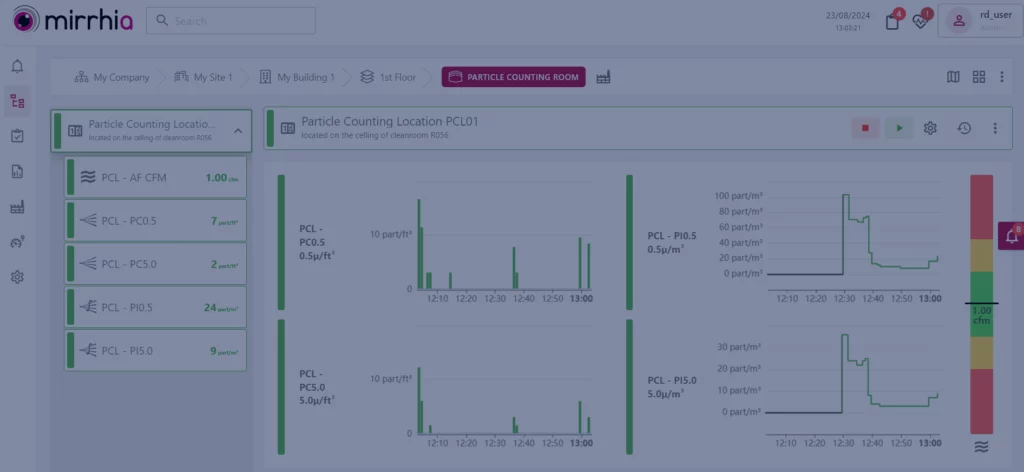
Mirrhia 2.5 is there!

Omega, Mirrhia’s Italian partner, in the spotlight in Genova Impresa

Arrival of Etienne Van den Bogaert as Managing Director
Mirrhia 2.4 is coming

Data Integrity in The Pharmaceutical Industry

Laborama 2024

Laboratory Temperature & Humidity Monitoring

Particle Counters | Case Studies & EMS in Pharma
