Annex 1 – New version
It’s been more than 3 months since the release of the new version of the Annex 1 and of course we thought we’d talk a bit about it 🙂
What is Annex 1 again?
As a reminder, this thin 59-page document, published by the European Commission, provides technical advice on the principles and guidelines of Good Manufacturing Practice (GMP) for human and veterinary medicinal products, both on the control and design of buildings, equipment, systems used and on procedures. All this, to avoid any contamination.
This Annex supports the authorities in the implementation of European laws. If you want to learn more about the Annex 1 and how it is structured, for example, I invite you to read an article I wrote with love entitled: Annex 1 for Dummies.
The revision of Annex 1, a long-awaited revision.
But why make a big deal out of it?
Well, I guess some people in the industry have been waiting for this since the dawn of time, or almost. The status of the document says it all: “Revision of the 2007 version of Annex 1”. And it’s almost 2023! You see what I mean? Anyway, it’s about time.
Well, in 16 years, much has happened since then, and the regulations have changed, the environments in which all these products are manufactured have seriously evolved.
In this new version, it’s the will to remove all ambiguity and inconsistencies that has taken over and so much the better. In the end, we don’t really want to play with people’s lives. And, the lightning evolution of the technologies used had to be taken into consideration.
A silly question…
When will it come into force?
August 25, 2023, which gives you 9 months if you need to change something in your company.
What changes, basically?
First of all, and frankly, this is a really important point, it is more understandable by the average person. Indeed, if the goal is to save lives, you’d better dictate rules that are understood by everyone, from the CEO of your pharmaceutical company to your cleanroom operator.
I have gobbled up the whole of Annex 1. Super interesting. And honestly yes, there was quite an effort in the writing!
If some paragraphs have disappeared, some have been doubled or even tripled. Some topics have been developed point by point, like the use of gloves, the environment, decontamination methods, cleaning and disinfection, etc…
The good news is that the structure remains the same. But one title has changed (which concerns us as a supplier of environmental monitoring solutions). Chapter 9 is now entitled: “Monitoring of the environment and processes” – instead of “Monitoring of the environment and viable and non-viable processes”.
Quality Risk Management throughout Annex 1 (QRM)
Quality Risk Management (QRM) is not new to Annex 1, but in this new version you are told from the start that QRM applies throughout the document and that you will find references and foundations all along.
A new section on Contamination Control Strategy (CCS)
One of the most important new requirements in the new Annex 1 is the Contamination Control Strategy (CCS).
The CCS should be implemented in all facilities to determine the critical control points for contamination and to evaluate the effectiveness of all these controls and monitoring measures. This is what will manage the risk to safety and product quality.
So let’s be clear, if you create a CCS and put it in a dusty drawer and forget about it for 16 years or forever, you are clearly not on the right track and neither is your sterile product production.
Your CCS is meant to be reviewed when necessary and updated regularly. The aim is to continuously improve the control methods. It should therefore be reviewed periodically, which will lead to updates in the pharmaceutical quality system (PQS).
A word to the wise: before changing anything, you must assess the impact of the change you want to make.
it is up to you to take all the necessary precautions to ensure that your product is indeed sterile.
The concept of RABS (Restricted Access Barrier Systems) or isolators
This was not present before. RABS or Isolators are those pieces of equipment that allow to minimize the microbial contamination linked to direct human interventions in critical areas (grade A). Have you ever seen these machines with integrated gloves? Well, that’s a RABS or isolator.
And what is the point of that? Well, these technologies ensure optimal conditions for products when they are in critical areas throughout the process and reduce the risk of contamination transfer. Note here that the material used for gloves, whether for isolators or RABS, must demonstrate sufficient chemical and mechanical resistance. And of course, the replacement frequency must be included in the CCS. If you use nice blue gloves from with RABS in a grade A area, they must be sterilized before use or bio-decontaminated!
What about environmental monitoring?
So, you can imagine that from the moment we manufacture a sterile drug that we are going to administer orally or by injection, we can’t do whatever we want and that the conditions in which we manufacture and package these products must be hyper controlled and systematically.
And the big change in this revision is the microbiological monitoring. It is clearly in your interest to review your environmental monitoring program to make sure that your operation is still in compliance with the legislation.
The new annex 1 states that a sampling plan must be written and that your contamination control strategy must directly influence your environmental monitoring program. This plan must provide you with all the data to ensure that your cleanrooms are truly clean and that you are able to detect excursions that could be a risk for the quality of your product.
With this reinforced Contamination Control Strategy (CCS), which will require to monitor your critical parameters, we’s like to advise you to get an environmental monitoring system like Mirrhia. Thanks to Mirrhia, you will be able to monitor and measure everything you want (particle count, pressure, etc.) and above all to display the trends to get a full control over your environment.
Now that the final version of Annex 1 has been published, it is important to review your environmental monitoring program to ensure that the methods used are still compliant.
What does this mean in practice? Based on a risk analysis and the results of this analysis, it is determined that an area or location should be monitored systematically and, if possible, in activity. (That is not always feasible).
For a class A area (basically, it’s a critical area for everything that is aseptic, filling vials for example), particle and microbiological monitoring must be carried out throughout the critical stages (this is where you will need MIRRHIA FACILITY®).
It is considered that the B zones (which you usually pass through before entering an A zone) should also be monitored like the A classes with less frequent sampling.
For the monitoring of classes C (e.g., where filter solutions are prepared and weighed) and D (e.g., where equipment is cleaned, for example), reference is made to the principles of quality risk management. The requirements, alerts and action thresholds depend on the nature of the operations performed.
This is not really a change to environmental monitoring but a strengthening of the contamination control strategy. As mentioned above: before changing anything, it is essential to assess the impact of the change you want to make… but above all to translate it into a process that you’ll be able to measure with Mirrhia.
A desire to harmonize
Between Annex 1, the WHO standards, the PIC/S (aka Pharmaceutical Inspection Cooperation Scheme), it was also time to harmonize all these principles related to the manufacture of sterile medicines. There was a will to be aligned with the FDA and its 2004 guidelines on sterile
Do you have a question on Annex 1?
Don’t hesitate to contact us now. Send us a message and we’ll get back to you shortly.
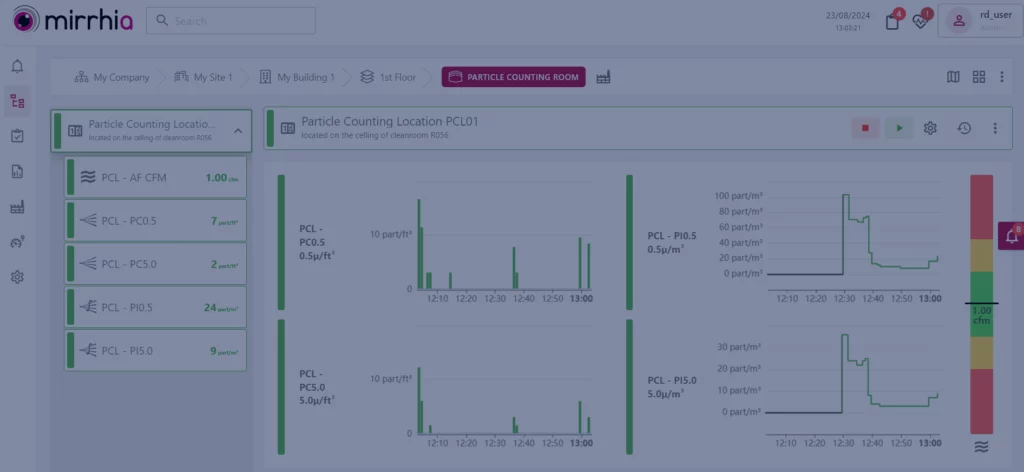
Mirrhia 2.5 is there!

Omega, Mirrhia’s Italian partner, in the spotlight in Genova Impresa

Arrival of Etienne Van den Bogaert as Managing Director
Mirrhia 2.4 is coming

Data Integrity in The Pharmaceutical Industry

Laborama 2024

Laboratory Temperature & Humidity Monitoring

Particle Counters | Case Studies & EMS in Pharma
