Environemental Monitoring – Glossary
I remember my first day of training to Mirrhia, our environmental monitoring solution. I scribbled some notes on a notebook, tons of acronyms and abbreviations that I had never heard in my life and that I googled for weeks.
This is the reason why I came up with the idea of creating a short list for you with these abbreviations and as I am pretty sure that more of these will pop up in the future, I will fill it up.
Let’s go!
EMS – Environmental Monitoring System
Alright, this one is easy. Environmental monitoring system. We are referring here to the monitoring of critical chemical or physical parameters using probes connected in RF, Wi-Fi or wired to a platform that displays and stores your data, alerts you in the event of an overrun, provides you with reports, etc. An EMS is generally qualified. Obviously when you work for pharma, the systems must be qualified. A kind of validity and security of your system that assures your client that everything is under control!
Discover our Environmental Monitoring Solutions
FMS – Facility Monitoring System
Yes, it is the same as EMS. As simple as that!
BMS – Building Management System
The BMS is not the same as an EMS. It is a building or site management system. The BMS monitors and controls, this is the big difference with an EMS which only monitors. Your BMS can therefore act as an EMS.
“Hooray” are you going to shout to me while running like a little madman through the corridors with your whistle. Now, you’re thinking that you will take a BMS, that it will cost you less and that it is better to avoid multiplication of systems.
Well, I’m sorry but I’m gonna say no. I don’t want to disappoint you but yes, it’s better to separate your EMS from your BMS. If you want to know why, read this article which will explain the differences between BMS and EMS.
BAS – Building Automation System
You might also hear about BAS instead of BMS. We are in a world where we seek to minimize human error and automate as many processes as possible. So it makes sense to talk about Building Automation System.
BCS – Building Control System
I know, there are quite a few different denominations to name one tool. Look in Wallonia, there’re a couple of terms used to talk about sweets… You can say bonbon, chic, mocks, and certainly other terms that I don’t know, straight out from outsliplous-les-bains-de-pieds…
BCS another term equivalent to BMS.
QBMS – Qualified Building Management System
Here is an abbreviation that interests us. In some countries, we speak of QBMS or Qualified Building Management System. Indeed, one of the essential information that a pharmaceutical company, a laboratory, certain departments of a hospital or a company that is under GMP constraint is that it must be equipped with a qualified environmental monitoring system. So, a QBMS is a qualified BMS.
QBAS – Qualified Building Automation System
Same thing here. BAS (Building Automation System) does exactly the same job as a BMS with the Q for “qualified”, we get a qualified BMS.
We can ask ourselves whether it is really appropriate for the entire BMS to be qualified. From our point of view, it is preferable to have an EMS (which will be qualified) separate from the BMS. I invite you to read our article EMS vs BMS (https://mirrhia.com/en/ems-bms/)
MES – Manufacturing Execution System
You may also hear of the term MES for Manufacturing Execution System. It is a program that controls and synchronizes the execution of processes related to the transformation of raw materials into intermediate or finished products. The MES also ensures complete traceability of these processes and delivers in electronic form the complete record of a production batch better known as the Electronic Batch Record (EBR). The EMS can therefore feed your MES to build a complete EBR integrating environmental data.
SCADA – Supervisory Control and Data Acquisition
If you work in a engineering office on the automation side, you must surely know this good old friend SCADA (or Supervisory Control and Data Acquisition).
SCADA is a technology that allows actions to be carried out through commands from a control platform. The aim is to reduce manual interventions in industrial installations. SCADA systems are mainly dedicated to monitoring and alarms. This is why you find this notion on this page.
And if you are wondering which one is better between SCADA and Mirrhia? I will answer that Mirrhia is a robust and packaged solution, a real advantage in terms of cost (license fees), time (little or no custom development, less qualification effort, etc.) compared to a SCADA.
By the way, I wrote this little article on SCADA, just for you!
Annex 1
The Annex 1 is a bit like your survival manual in the jungle, a manual written by Tarzan. What he tells you to do to survive, you do it.
Well, that’s a bit like Annex 1, if you want to manufacture a sterile medicine and market it, you must follow a certain number of rules which will ensure that, at the end of the chain, your medicine will be sold and used without danger. This involves a whole series of requirements regarding quality, hygiene, safety, and environment conditions in the pharmaceutical industry. These requirements are listed in Annex 1.
And if you feel like it, I had a lot of fun writing an article on the Annex 1 for dummies.
21CFR Part11
21CFR part11 is a guidance, created in 1997 by the FDA (Food and Drug Administration) in the USA. This document establishes the rules to be followed in order to ensure data traceability (Data Integrity) and electronic document management. The standard is intended for pharmaceutical, cosmetic and food companies that want to market their products in the United States.
Why 21? Why CFR? 21 for Title 21 of the Code of Federal Regulations – CFR.
Its European equivalent is Annex 11. Annex 11 is a directive on Good Manufacturing Practices (GMP) in the European Union.
in both cases, we clearly want to ensure the accuracy, authenticity and reliability of the programs and systems that process digital data.
GMP – Good Manufacturing Practice
The FDA requires compliance with Good Manufacturing Practices (GMP) to ensure that products manufactured by manufacturers meet its quality requirements.
So, when we talk about GMP, we are talking about code of conduct or advice regarding the manufacture and quality assurance of products. The ultimate goal here is to protect the end user, human beings, and to guarantee that the product is safe and suitable for consumption.
The EMS (Environmental Monitoring System) meets GMP standards.
GEP – Good Engineering Practice
This is a set of specifications, rules, advice, and methods regarding pharmaceutical and biotech facilities and buildings, both in terms of their design and construction, operation, or management. The BMS (Building Management System) meets GEP standards.
GAMP – Good Automated Manufacturing Practice
GAMP is a guide for the design and validation of computer systems. “GAMP5: A Risk-Based Approach to Compliant GxP Computerized Systems” is a document published by ISPE that provides a framework. It is therefore not a regulation but a guideline.
Mirrhia is GAMP5 category 4, this means that our software is configured and pre-qualified. The functional requirements tests have already been carried out. On-site qualification will therefore be reduced. And that… is beautiful!
A question, an info?
Don’t hesitate to contact us now. Send us a message and we’ll get back to you shortly.
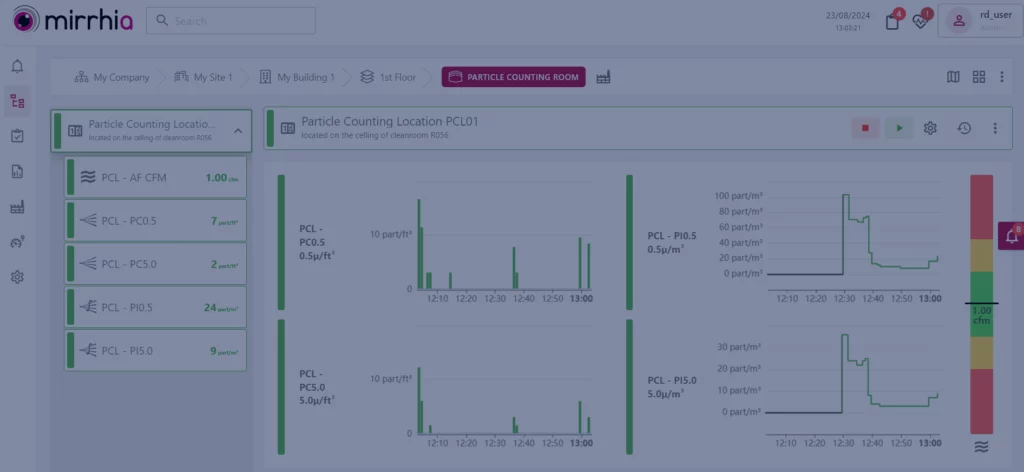
Mirrhia 2.5 is there!

Omega, Mirrhia’s Italian partner, in the spotlight in Genova Impresa

Arrival of Etienne Van den Bogaert as Managing Director
Mirrhia 2.4 is coming

Data Integrity in The Pharmaceutical Industry

Laborama 2024

Laboratory Temperature & Humidity Monitoring

Particle Counters | Case Studies & EMS in Pharma
